Achalasia
Types:
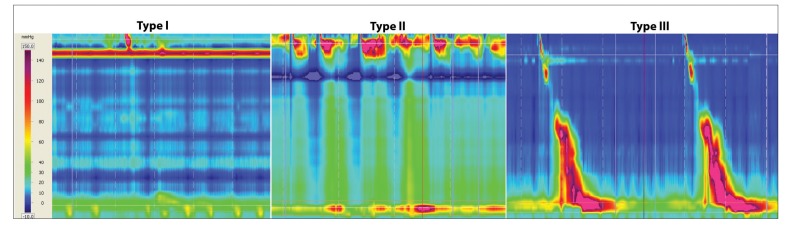
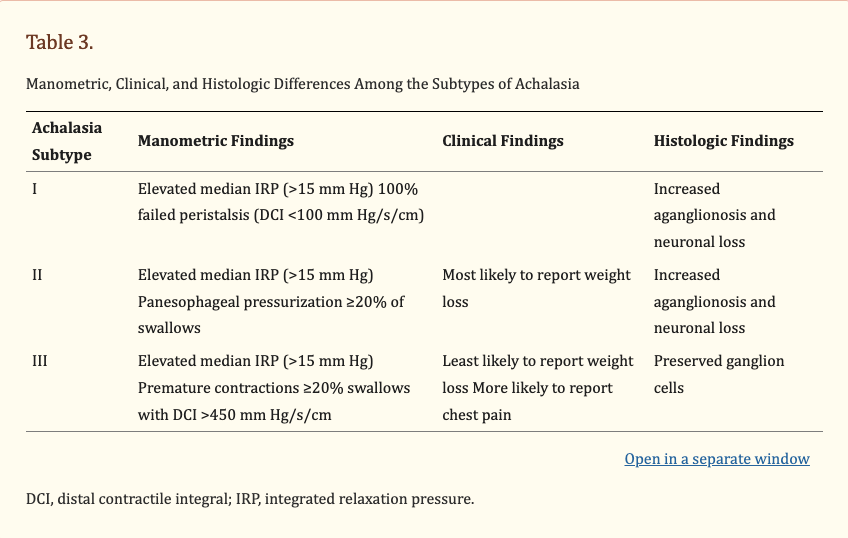
Type I – minimal contractility in oesophageal body
Type II – intermittent periods with panoesophageal pressurisation
Type III – (spastic) with premature of spastic distal oesophageal contractions
Management should be with Heller’s cardiomyotomy or pneumatic dilatation.
Unfit patients may benefit from botulinum toxin injection
Calcium channel blockers (Nifedipine 10-30mg, given 30-45 minutes before meals) can reduce LES resting pressure by 13-49% and is effective in 75% of patients. However, the side effects (headaches, orthostatic hypotension, lower limb oedema) in 30% mean it should be used as a bridge to more definitive therapy.
Other medical possibilities are sublingual nitrates (more severe side effects), anticholinergics (atropine, cimetropium bromide), B-agonists and even Sildenafil.
Greek meaning: ‘failure to relax’
Defined as failure of peristalsis with failure of the lower oesophageal sphincter to relax completely. There are both primary and secondary achalasia.
Primary achalasia
Occurs in less than 1 per 100 000. There is progressive loss of ganglion cells in the myenteric plexus. Possibly due to inflammation due to its presence in the surrounding tissues, however a definite connection with viral or autoimmune aetiology hasn’t been found.
Patients present with dysphagia, often in middle age, that affects both liquids and solids. Often have chest pain. Will have been slow eaters for years, having developed tricks to aid swallowing.
Important to assess the respiratory system (recurrent chest infections) and nutritional status (although outright malnutrition is rare given that after the oesophagus fills enough it eventually overcomes the pressure of the LOS)
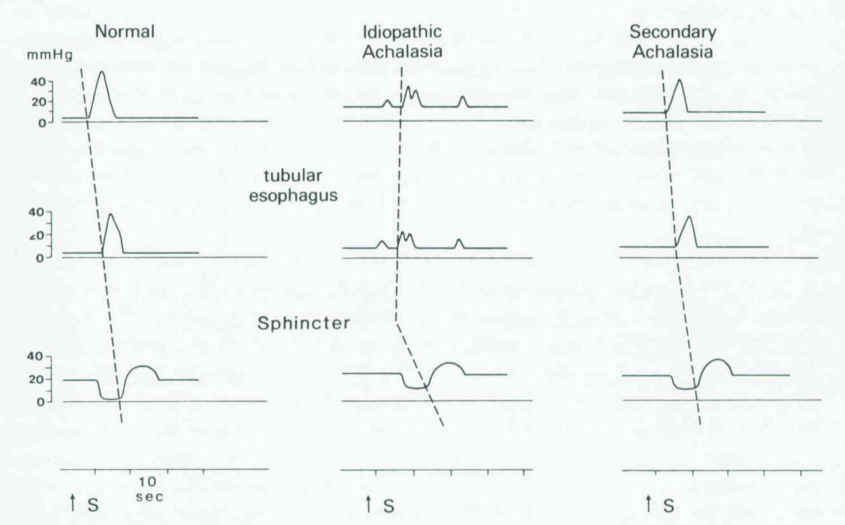
Investigations are with OGD (food residue in oesophagus, tight cardia) and Ba Swallow (bird’s beak, absence of air bubble; but often normal until late in the disease).
But oesophageal manometry is needed to confirm the diagnosis: hypertensive LOS that doesn’t relax fully, aperistalsis of the oesophageal body, raised resting pressure in the oesophagus.
Treatment is with either dilatation, cardiomyotomy or botulinum injections
– Pneumatic dilatation: stretching the cardia with a balloon to disrupt the muscle fibers. First described by Plummer in 1908. New balloons have a precise pressure and diameter and will split length ways if pressure is too high. The diameter of the balloons are 30-40mm. with the 30mm balloon the risk of perforation should be <0.5% when done cautiously over several weeks. Outcomes can be symptom relief in for over 1 year in 70-90% of patients.
– Cardiomyotomy : Heller initially did an anterior and posterior myotomy but nowadays we just do an anterior. Supposedly, reducing the gastric myotomy to 1cm reduces GORD but a Dor anti-reflux procedure has been shown to reduce GORD from 48% to 9% (pH study). As the oesophagus is often aperistaltic, a complete wrap wouldn’t be appropriate.
2 randomised trials have found resolution of dysphagia to be more effective after myotomy compared to dilatation. But GORD is more common.
QALY studies find dilatation and surgery to be equally effective but cost-analysis favours dilatation.
The effect of previous treatments remains contested, with 2 large case-series in Europe reaching different conclusions.
Cancer risk: 30-40 fold increase risk of squamous cancer (well recognised), 10-fold increase of adenocarcinoma in men (recent Swedish study)
Secondary achalasia
Chagas’ disease (Trypanosoma Cruzi) common in South America, causes a mega-oesophagus and chronic oesophagitis with retention. Cardiomyopathy is the cause of death but some will require oesophagectomy.
Psuedo-achalisa is similar symptoms caused by adenocarcinoma or GISTs at the LOS. Also by a para-neoplastic process from lesions in the lung or panceas.
After fundoplication, if the wrap is too tight it can cause similar symptoms.
Heller’s cardiomyotomy
NG tube
Catheter
Patient in lithotomy position
Surgeon stands between the legs
Incision:
Initial trocar 13-18cm from xyphoid process in midline
3-4 additional ports – liver retractor, 2 working ports, 1 assistant port
Procedure:
A nathienson’s retractor holds the left lobe of the liver up
The gastrohepatic ligament (pars flaccida) is divided, followed by division of the oesophago-phrenic ligaments. Start the division at the transverse fat pad that marks the decussation of the crus fibers.
IDENTIFY ANTERIOR VAGUS NERVE AND PRESERVE
Make a retro-oesophageal window and pass a catheter, clip with large ligaclip. Use this for retraction.
Myotomy – Identify GOJ, by caudal retraction on gastroesophageal fat pad or the sling. Start by using Maryland to open fibers into the right plane. Start on the stomach side as this is the more difficult side. Use the ligasure to sweep left and right to open up the plane. If you’re in the right plane, this should be easy, you shouldn’t need to spread the jaws. Then divide the longitudinal muscle fibers above the plane, carry on up the oesophagus for 6cm. Continue the division for 2-3cm on the stomach, down to the first branch on the lesser curve.
Perform airleak test or do endoscopy, insufflate the stomach and see where fibers are causing a kink. Use hook to divide these fibers.
Divide the adhesions to spleen on the fundal side then through the retro-oesophageal window, use a sweeping motion to divide the adhesions to the left crus. This should mobilised enough stomach to make the wrap.
Perform a Dor (anterior) fundoplication: Stitch the myotomy on both sides to the crus. Then place three stitches to ‘cover the evidence’ ie. stitch the mobilised stomach to the right crus to close the hiatus.